Which of the Following Atoms Is Not a Halogen
Solutions for Chapter 4B Problem 14T. Which of the following atoms does not participate in hydrogen bonding.
H 2 is not pseudo halogen compound.

. Since its upload it has received 173 views. 22 Which of the following is not part of atomic theory. A halogen is a chemical element that forms a salt when it reacts with metal.
D Chain termination occurs when two readicals react with each o. Asked Feb 27 2020 in Trades Technology by helpmeout. Protons would be in Group 17 of the periodic table which means atomic number 53 is iodine and for B a noble gas with a is equal the 84 a represents the mass number and a noble gas of the mass number or an atomic mass.
C Atoms of one element can be changed to atoms of another element in a chemical reaction. Halogens are those elements which have 7 electrons in their outer shell. A All matter is composed of small indivisible particles called atoms.
The covalent bond is formed by. Haloalkanes are a class of chemical compounds that comprise of an alkane with one or more hydrogen atoms replaced by halogen atoms. D When HX adds to an asymmetrical alkene the halogen attaches to the least substituted sp² carbon.
Halogens are a family of atoms the penultimate column of the periodic table. A S B O C F D N 29. Glycerine is also known as glycerol it is a viscous liquid which is made of palm oil or soybean.
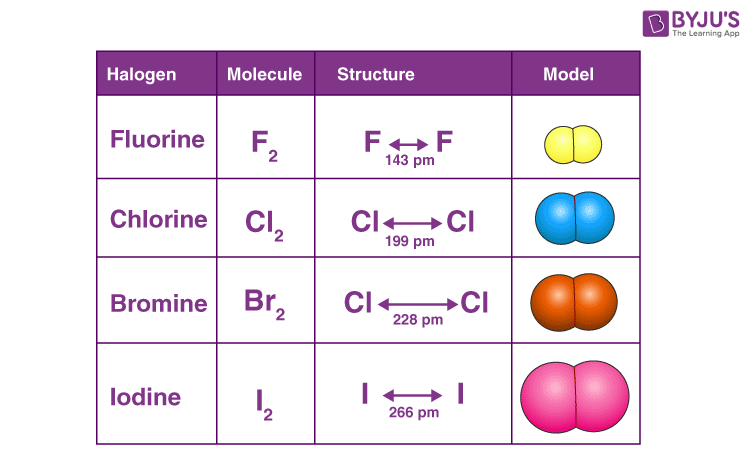
Each molecule contains two halogen atoms. C Hydrogen atoms are produced in the first propogation step. Which of the following is pseudo halogen compounds.
Of these chlorine and iodine are essential for human nutrition although the other elements might also be required in trace amounts. What are halogen atoms. It reacts all the other elements except the lighter noble gars He Ne and Ar.
Glycerine is also known as glycerol it is a viscous liquid which is made of palm oil or soybean. 1 magnesium atom 2 nitrogen atoms and 4 oxygen atoms. Glycerine is not a halogen.
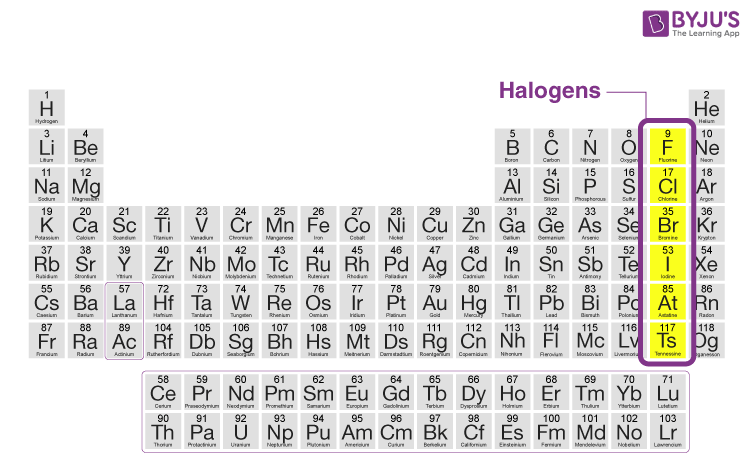
The halogen elements are fluorine F chlorine Cl bromine Br iodine I astatine At and tennessine Ts. It reacts with Xe under mild condition to form Xe fluorides. Theyre composed of Fluorine Bromine Chloride Iodine and Astatine.
Silicon belongs to Group IVA. The artificially created element 117 tennessine may also be a halogen. Accepting electron pair by one atom.
These elements are bromine iodine fluorine astatine and chlorine. Halogens are those elements which have 7 electrons in their outer shell. Complete transfer of electrons.
The halogen derivatives of aromatic hydrocarbons having the halogen atom directly attached to a carbon atom of the aromatic ring are called haloarenes. What is the classification of Haloalkanes. It has ______ electrons in the valence shell.
In the assive form a few metals such as Cu Ni Fe and Al acquire a protective fluoride coating. 2 sodium atoms 1 sulfur atom and 4 oxygen atoms. Fluorine chlorine bromine iodine and astatine.
Lets identify the following Adams for a hey login with 53 electrons would also have 53. Which of the following is pseudo halogen compounds. In the modern IUPAC nomenclature this group is known as group 17.
Mutual sharing of an electron pair by two atoms. Which of the following compounds is not an interpseudo halogen. Glycerine is not a halogen.
1 Rank the following halogen atoms in order of selectivity from 1 most selective to 3 least selective. Donating an electron pair by one atom. This is known as Markovnikovs rule.
Which of the following substances would have the highest critical temperature. Which of the following statements about the reaction is not truea Halogen atoms are consumed in the first propagation stepb Halogen atoms are regenerated in the second propagation stepc Hydrogen atoms are produced in the first propagation stepd Chain termination occurs when two radicals react with each other. These are fluorine chlorine bromine and iodine.
Asked Nov 17 2020 in Physics Space Science by jenacondron. 1 Rank the following halogen atoms in order of selectivity from 1 most selective to 3 least selective. Joined by a single covalent bond.
The question contains content related to Chemistry and Science. 1 aluminum atom and 3 bromine atoms. Reations of F2 with many element s are vigorous and ofter explosives.
The pseudohalogens are polyatomic analogues of halogens whose chemistry resembling that of the true halogens allows them to substitute for halogens. Which of the following statements about the reaction is not true. On StudySoup on 5312017.
The table shows the colour and physical states. Which of the following is not a halogen. Write a chemical formula for each molecular model.
The halogens are a group in the periodic table consisting of five or six chemically related elements. A When HX adds to an asymmetrical alkene the sp² carbon bonded to the most H atoms is protonated. The word halogen means salt former.
Halogen any of the six nonmetallic elements that constitute Group 17 Group VIIa of the periodic table. Each halogen atom such as chlorine fluorine and bromine in Earths atmosphere contributes to. Which of the following statements is NOT true.
B All atoms of a given element have identical mass and chemical properties. When halogens react with metals they. Taking intermolecular forces into account which halogen wouldyou expect to have the highest boiling.
Of chlorine bromine and iodine at room temperature. Which of the following is not an inter - halogen compound. 2 lithium atoms 1 sulfur atom and 4 oxygen atoms.
This 12 words question was answered by Heather L. A CHCI B CH C F D H E CO Page 6. A Halogen atoms are consumed in the first propogation step b Halogen atoms are regenerated in second propogating step.
The lighter halogens occur in living organisms. They have a general formula of P s P s or P s X where P s is a pseudohalogen group such as cyanogen pseudohalide anions such as cyanide ion. Fluorine is the nost reactive of all the elements in the periodic table.
A halide torch will only detect products containing halogen compounds.

Halogen Elements List And Facts

Printable Periodic Table And Atom Modeling Atom Model Periodic Table Atom

Collective Names Of Like Elements Chemicalelements Periodictable Halogens Pnictogens Chalcogens Alkali Chemistry Education Chemistry Notes Physics Notes
Which Group Does Astatine Belong To Nonmetal Halogen Or Noble Gas Quora

New Periodic Table Groups Color Coded Tablepriodic Priodic Tablepriodicsample Chemistry Worksheets Science Worksheets Science Notes

Tennessine Chemistry Experiments Middle School Science Experiments Science Experiments Kids Preschool
Halogens Definition Uses Compounds Properties Of Halogens

Prologue Chemistry Notes Bullet Journal Ideas Chemistry Notes Bullet Journal Chemistry

Electronic Configuration Halogen Characterisitcs Periodic Table

Tennessine Chemistry Experiments Middle School Science Experiments Science Experiments Kids Preschool

In Interhalogen Compounds Which Of The Following Halogens Is Never The Central Atom

Pinterest High Pressure Halogen Color
Halogens Definition Uses Compounds Properties Of Halogens
Halogens Fluorine Chlorine Bromine Iodine Astatine

Hypothetical Periodic Table Google Search Periodic Table Internet Database Save

Comparing Halogen Reactivity Trends With Those Of The Alkali Metals Halogens Become Less Reactive Down The Chemistry Classroom High School Chemistry Chemistry



Comments
Post a Comment